The Role of Methylation in Breast Cancer Evolution and Development
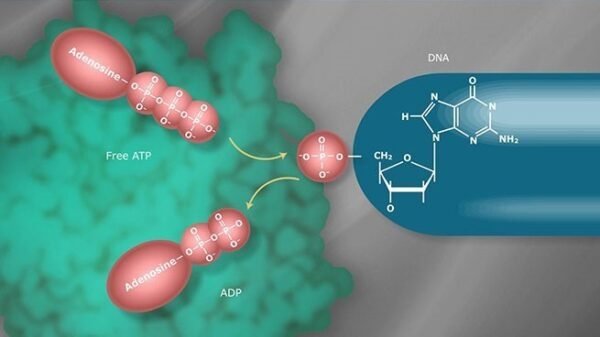
Methylation is an essential epigenetic mechanism involving the addition of a methyl group to DNA, often impacting gene expression without altering the underlying genetic sequence. In the context of breast cancer, aberrant DNA methylation patterns have been identified as critical factors in tumor initiation, progression, and response to treatment. Understanding methylation’s role in breast cancer evolution offers new insights into the disease’s biology and highlights potential avenues for diagnosis and therapy.
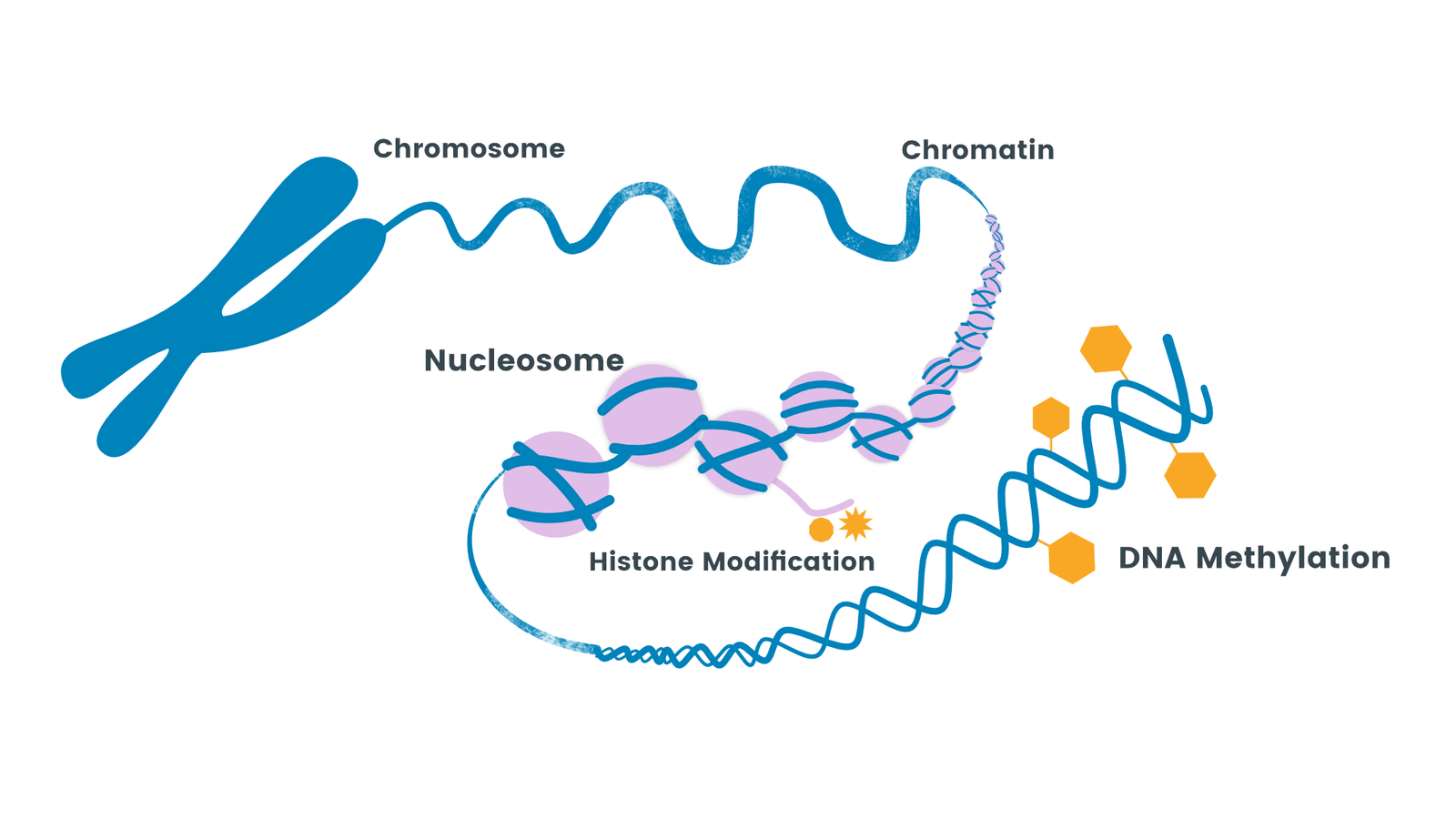
DNA Methylation and Gene Regulation
In healthy cells, DNA methylation typically occurs at CpG islands—regions rich in cytosine and guanine nucleotides—found near gene promoters. When these regions are methylated, gene expression is usually silenced. Conversely, unmethylated CpG islands tend to be associated with active gene transcription. In breast cancer, however, this regulation becomes disrupted, leading to inappropriate activation or silencing of key genes involved in cell growth, apoptosis, and DNA repair.
Hypermethylation of Tumor Suppressor Genes
One of the most well-documented roles of methylation in breast cancer is the hypermethylation of tumor suppressor genes. Genes such as BRCA1, p16, and RASSF1A, which help regulate cell cycle arrest and apoptosis, are frequently found to be hypermethylated in breast cancer tissues. This epigenetic silencing prevents these crucial genes from functioning properly, contributing to uncontrolled cell division and the onset of malignancy.
Hypomethylation and Oncogene Activation
In addition to hypermethylation of tumor suppressors, hypomethylation of oncogenes plays a significant role in breast cancer. Hypomethylation, or the loss of methyl groups, often occurs in repeat sequences and can lead to genomic instability. It can also result in the activation of proto-oncogenes such as MYC and RAB39A, further driving tumor development. This hypomethylation-associated instability may enable breast cancer cells to acquire new mutations, promoting the evolution and progression of the tumor.
Methylation and Breast Cancer Subtypes
Different subtypes of breast cancer exhibit distinct methylation patterns. For instance, triple-negative breast cancer (TNBC), an aggressive form of the disease, is often characterized by widespread methylation changes, particularly affecting immune response genes. These methylation alterations contribute to TNBC’s rapid progression and poor prognosis. In contrast, hormone receptor-positive breast cancers may have different methylation profiles, reflecting the heterogeneity of the disease.
Role in Tumor Microenvironment and Immune Evasion
Methylation also affects the breast cancer tumor microenvironment, particularly in how tumors evade immune surveillance. Aberrant methylation can silence genes involved in antigen presentation and immune recognition, allowing cancer cells to avoid detection by the immune system. Additionally, changes in the methylation status of certain immune-modulating genes can alter the tumor’s interaction with immune cells, enabling the tumor to create a more permissive environment for its growth.
Methylation as a Diagnostic and Therapeutic Target
Given its significant role in breast cancer development, methylation serves as both a diagnostic marker and a potential therapeutic target. Specific methylation patterns can be detected in blood or tissue samples, offering a non-invasive way to screen for breast cancer or predict its recurrence. Additionally, drugs known as DNA methyltransferase inhibitors (DNMT inhibitors), such as azacitidine and decitabine, have been explored as treatments to reverse abnormal methylation patterns and restore normal gene function in cancer cells.
Conclusion
Methylation plays a crucial role in the evolution and development of breast cancer, affecting tumor suppressor gene silencing, oncogene activation, and interactions with the immune system. By understanding and targeting these methylation changes, clinicians can improve breast cancer detection, prognosis, and treatment outcomes. Future research into methylation-based therapies holds promise for more personalized and effective breast cancer interventions.



Leave a Reply