Targeting Phosphorylation: Advances in Breast Cancer Therapy
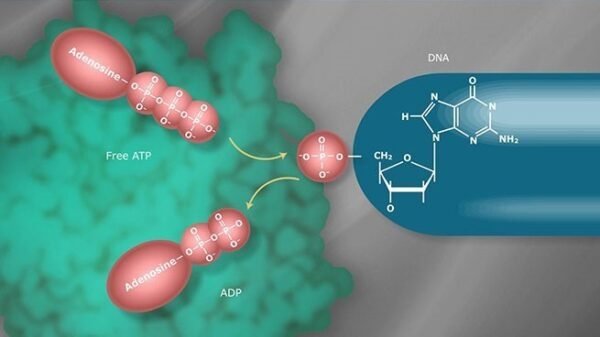
Phosphorylation is a critical post-translational modification that plays a central role in regulating signaling pathways involved in the development and progression of breast cancer. It involves the addition of a phosphate group to proteins, typically by kinases, which can activate or deactivate these proteins and modulate cellular functions such as growth, survival, and apoptosis.
In breast cancer, phosphorylation regulates key signaling cascades such as the PI3K/AKT/mTOR and MAPK pathways, both of which are often dysregulated. Overexpression or hyperactivation of kinases like HER2/EGFR, which drive these pathways, can lead to uncontrolled cell proliferation and tumor growth. Mutations in kinases, phosphatases (enzymes that remove phosphate groups), or their regulators can also contribute to aberrant signaling, leading to resistance to therapy and disease progression.
Moreover, phosphorylation serves as a critical mechanism for drug resistance, particularly in hormone receptor-positive breast cancers. Hormone receptors, such as estrogen and progesterone receptors, can be phosphorylated, altering their activity and influencing the cancer’s responsiveness to endocrine therapies.
Given its central role, targeting phosphorylation events through kinase inhibitors has become a prominent strategy in breast cancer treatment. Drugs like HER2 inhibitors (e.g., trastuzumab) or CDK4/6 inhibitors aim to disrupt the aberrant signaling that is sustained by abnormal phosphorylation, offering targeted therapeutic options for patients.
In summary, phosphorylation is a key regulator of breast cancer signaling pathways, influencing tumor development, progression, and response to treatment. Understanding these mechanisms is crucial for the development of targeted therapies and improving patient outcomes.



Leave a Reply