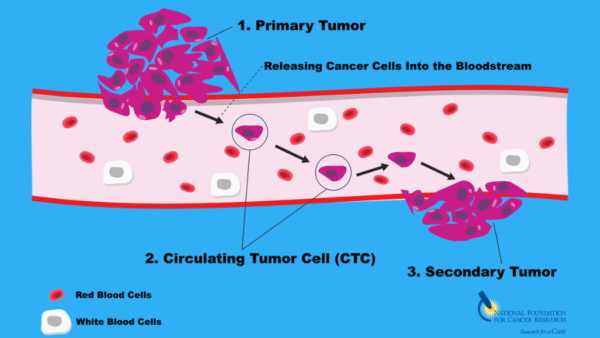
Circulating Tumor Cells (CTCs)
Circulating Tumor Cells (CTC) Testing for Early Metastases Detection
Circulating Tumor Cells are cancer cells shed from primary or metastatic tumors into the bloodstream. Unlike traditional biopsy-based methods, which rely on tissue samples, CTC testing offers a minimally invasive approach, capturing cancer’s systemic nature. These cells hold valuable information regarding tumor dynamics, genetic mutations, and potential treatment responses.
Understanding Circulating Tumor Cells (CTCs):
One of the most significant advancements in precision medicine is the use of biomarker testing. Biomarkers are molecules that indicate the presence of cancer and can provide information about the cancer’s characteristics. By identifying these biomarkers, doctors can select treatments that are most likely to be effective for a particular patient, avoiding a one-size-fits-all approach. This personalized strategy not only enhances treatment efficacy but also opens the door to new therapeutic options, including immunotherapy and targeted drug therapy.
Significance of Early Metastases Detection:
Metastasis represents a critical juncture in cancer progression, where cells migrate from primary sites to distant organs, initiating secondary tumor formation. Early detection of metastases significantly impacts treatment strategies, prognosis, and patient outcomes. By identifying CTCs, clinicians gain insight into the disease’s spread before overt metastases manifest clinically, enabling timely intervention.
Methodology of CTC Testing:
CTC testing employs various techniques to isolate and characterize these elusive cells from peripheral blood samples. Common methodologies include immunomagnetic separation, microfluidic devices, and size-based filtration systems. Once isolated, CTCs undergo molecular analysis, including genetic profiling, gene expression profiling, and drug sensitivity testing. Integration of advanced technologies such as next-generation sequencing enhances the sensitivity and specificity of CTC detection, facilitating personalized treatment strategies.
Implications in Clinical Practice:
The integration of CTC testing into clinical practice holds profound implications for cancer management. Firstly, it enables non-invasive monitoring of disease progression and treatment response, circumventing the limitations of conventional imaging modalities. Moreover, CTC-based molecular profiling guides therapeutic decision-making, allowing for targeted therapies tailored to an individual patient’s genetic makeup. Additionally, early detection of treatment-resistant CTC subpopulations prompts timely modifications in therapeutic regimens, preventing disease recurrence and improving long-term outcomes.
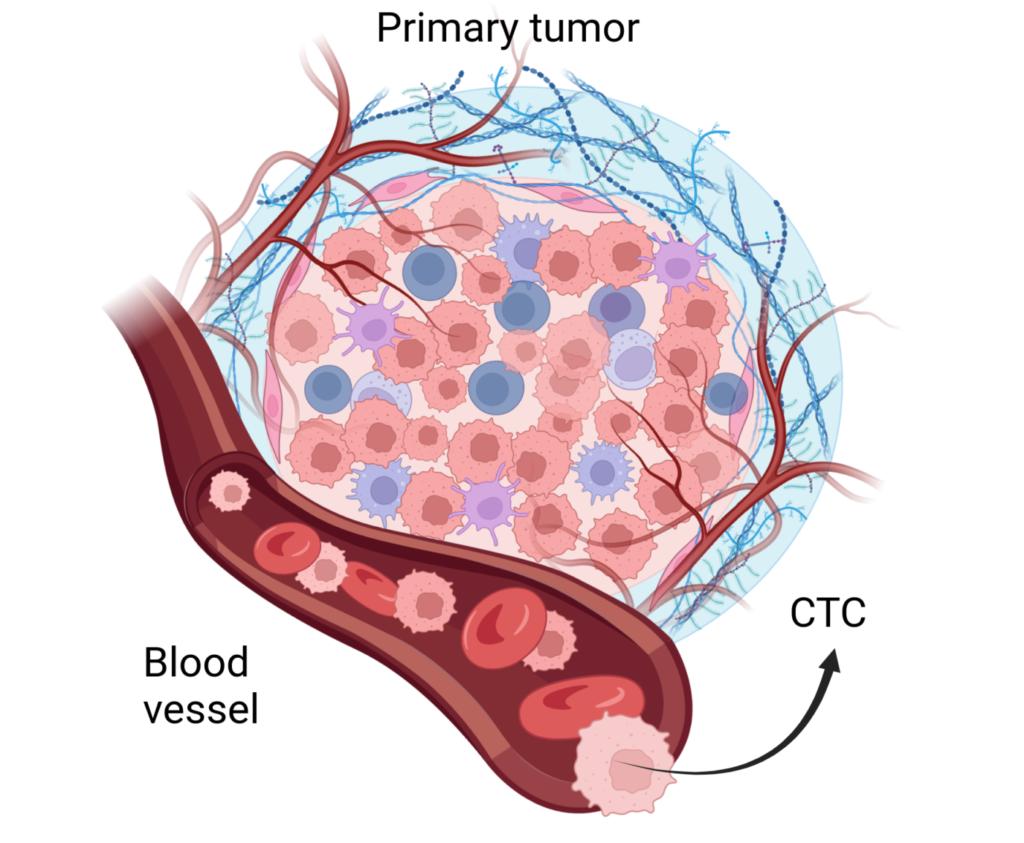
Challenges and Future Directions:
Despite its promise, CTC testing faces several challenges, including technical limitations, standardization issues, and cost considerations. Enhancing assay sensitivity and specificity, optimizing sample processing protocols, and establishing standardized analytical methodologies are imperative to overcome these obstacles. Moreover, large-scale clinical validation studies are warranted to demonstrate the clinical utility and cost-effectiveness of CTC testing across various cancer types and stages. Future research endeavors should focus on integrating CTC-based liquid biopsies into routine oncological practice, fostering collaborative efforts among researchers, clinicians, and industry stakeholders.
Case Studies:
Several clinical studies have demonstrated the clinical utility of CTC testing in various cancer types. For instance, in breast cancer, CTC enumeration serves as a prognostic marker for disease recurrence and survival outcomes. Similarly, in prostate cancer, the detection of androgen receptor splice variant-positive CTCs predicts resistance to hormonal therapy, guiding treatment decisions. Furthermore, in lung cancer, molecular profiling of CTCs identifies actionable mutations, guiding targeted therapy selection. These case studies underscore the versatility and efficacy of CTC testing across diverse malignancies.
Ethical Considerations:
The integration of CTC testing into clinical practice raises ethical considerations regarding patient consent, privacy protection, and equitable access to advanced diagnostics. Ensuring informed consent, safeguarding patient confidentiality, and promoting equitable distribution of resources are paramount. Additionally, addressing concerns regarding data ownership, interpretation, and utilization is essential to uphold ethical standards and maintain public trust in medical innovation.
Conclusion:
Circulating Tumor Cells testing represents a paradigm shift in cancer diagnosis and management, offering a non-invasive, real-time approach to detect early metastases and guide personalized treatment strategies. By harnessing the potential of liquid biopsies, clinicians can optimize patient care, improve treatment outcomes, and ultimately, transform the landscape of cancer care. As research advances and technology evolves, CTC testing holds the promise of revolutionizing cancer management, bringing us closer to the elusive goal of conquering this formidable disease.


Leave a Reply