From Genes to Therapy: Advances in Understanding Breast Cancer’s Genetic Drivers
Recent scientific research into the genetic causes of breast cancer has made significant strides in understanding how both inherited and acquired mutations influence tumor development, progression, and treatment. One of the key areas of focus has been identifying how specific genetic variations, particularly in oncogenes and tumor suppressor genes, shape different subtypes of breast cancer and impact a patient’s prognosis.
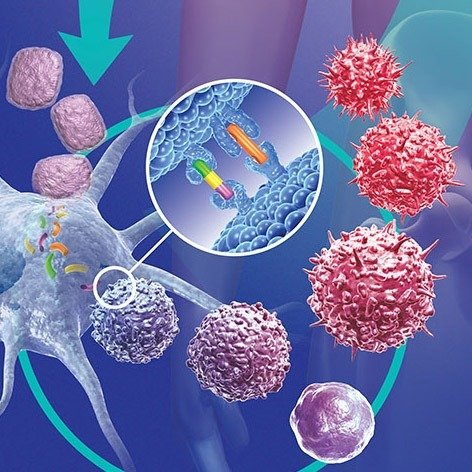
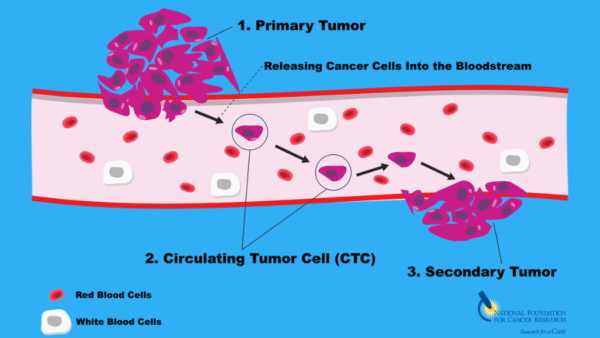
A prominent study explored the role of BRCA1 and BRCA2 mutations, which are well-known to increase the risk of breast cancer, particularly triple-negative breast cancer (TNBC). However, recent findings indicate that these inherited mutations may play a role beyond simply increasing susceptibility. Research has shown that these germline mutations influence the way breast cancer cells interact with the immune system, specifically how tumors evolve to evade immune detection. These mutations can lead to a phenomenon known as “germline-mediated immunoediting,” where early-stage tumors with specific genetic mutations are more susceptible to immune surveillance but, over time, develop mechanisms to escape detection and become more aggressive.
In addition to BRCA genes, other genetic drivers are being uncovered. Studies involving large populations of women with and without breast cancer have identified novel genetic variants that influence the likelihood of developing the disease. For instance, alterations in non-coding RNAs (lncRNAs), which were once considered “junk” DNA, have been found to play crucial roles in regulating gene expression in breast cancer. These discoveries are reshaping our understanding of how certain breast cancers develop, and they open new pathways for personalized treatment.
Moreover, the classification of breast cancer into subtypes has evolved with the integration of genetic data. By examining thousands of tumors, researchers have categorized breast cancer into 11 subtypes, each associated with different risks of recurrence and prognosis. These classifications are based on both somatic mutations acquired during a person’s life and inherited genetic factors, helping clinicians tailor treatment plans more precisely.
As a result of these genetic insights, new therapeutic approaches are being developed. Targeted therapies that exploit the specific vulnerabilities of cancer cells based on their genetic makeup are gaining prominence. Immunotherapies, for example, aim to “reignite” the immune system’s ability to recognize and destroy cancer cells, especially in cases where the tumors have developed mechanisms to evade immune responses.
In summary, the latest research highlights the complexity of breast cancer genetics, emphasizing the interplay between inherited mutations and tumor evolution. These findings are not only enhancing our ability to predict breast cancer risk but are also paving the way for more effective, personalized treatment strategies aimed at improving outcomes for breast cancer patients.


Leave a Reply